To See Etea result, Download The app.
Etea Result
Nomenclature
Alkyl Halide (Examples)
EXAMPLE 10.1.1
Give the common and IUPAC names for each compound.
- CH3CH2CH2Br
- (CH3)2CHCl
SOLUTION
- The alkyl group (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.
- The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the middle carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC name, the Cl atom (prefix chloro-) attached to the middle (second) carbon atom of a propane chain results in 2-chloropropane.
EXERCISE 10.1.1
Give common and IUPAC names for each compound.
- CH3CH2I
- CH3CH2CH2CH2F
Example 10.1.2
Give the IUPAC name for each compound.
SOLUTION
- The parent alkane has five carbon atoms in the longest continuous chain; it is pentane. A bromo (Br) group is attached to the second carbon atom of the chain. The IUPAC name is 2-bromopentane.
- The parent alkane is hexane. Methyl (CH3) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. Listing the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
EXERCISE 10.1.2
Give the IUPAC name for each compound.
The haloalkanes, also known as alkyl halides, are a group of chemical compounds comprised of an alkane with one or more hydrogens replaced by a halogen atom (fluorine, chlorine, bromine, or iodine). There is a fairly large distinction between the structural and physical properties of haloalkanes and the structural and physical properties of alkanes. As mentioned above, the structural differences are due to the replacement of one or more hydrogens with a halogen atom. The differences in physical properties are a result of factors such as electronegativity, bond length, bond strength, and molecular size.
Alkyl Halide
Alkyl halides are also known as haloalkanes. This page explains what they are and discusses their physical properties. alkyl halides are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). We will only look at compounds containing one halogen atom. For example:
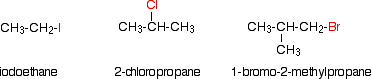
alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. There are some chemical differences between the various types.
Primary alkyl halides
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group.Some examples of primary alkyl halides include:
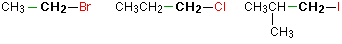
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the halogen. There is an exception to this: CH3Br and the other methyl halides are often counted as primary alkyl halides even though there are no alkyl groups attached to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. Examples:
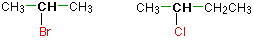
Tertiary alkyl halides
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
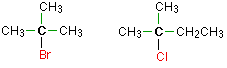
The Learning Objective is to name halogenated hydrocarbons given formulas and write formulas for these compounds given names.
Many organic compounds are closely related to the alkanes. As we noted in Section 12.7, alkanes react with halogens to produce halogenated hydrocarbons, the simplest of which have a single halogen atom substituted for a hydrogen atom of the alkane. Even more closely related are the cycloalkanes, compounds in which the carbon atoms are joined in a ring, or cyclic fashion.
The reactions of alkanes with halogens produce halogenated hydrocarbons, compounds in which one or more hydrogen atoms of a hydrocarbon have been replaced by halogen atoms:

The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by number indicating the substituent’s location. The prefixes are fluoro-, chloro-, bromo-, and iodo-. Thus CH3CH2Cl has the common name ethyl chloride and the IUPAC name chloroethane. Alkyl halides with simple alkyl groups (one to four carbon atoms) are often called by common names. Those with a larger number of carbon atoms are usually given IUPAC names.
Alkyne Naming Roles
Naming Alkynes
Like previously mentioned, the IUPAC rules are used for the naming of alkynes.
Rule 1
Find the longest carbon chain that includes both carbons of the triple bond.
Rule 2
Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes. For example:
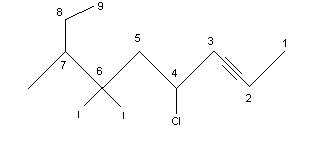.png?revision=1&size=bestfit&width=333&height=144)
Rule 3
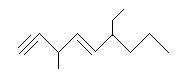
After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituent use the prefixes di, tri, and tetra for two, three, and four substituents respectively. These prefixes are not taken into account in the alphabetical order. For example:
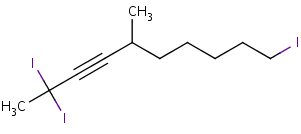
2,2,10-triiodo-5-methyl-3-decyne
If there is an alcohol present in the molecule, number the longest chain starting at the end closest to it, and follow the same rules. However, the suffix would be –ynol, because the alcohol group takes priority over the triple bond.
5- methyl-7-octyn-3-ol
When there are two triple bonds in the molecule, find the longest carbon chain including both the triple bonds. Number the longest chain starting at the end closest to the triple bond that appears first. The suffix that would be used to name this molecule would be –diyne. For example:
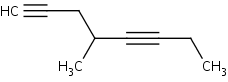
4-methyl-1,5-octadiyne
Rule 4
Substituents containing a triple bond are called alkynyl. For example:
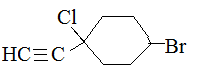.png?revision=1&size=bestfit&width=210&height=80)
1-chloro-1-ethynyl-4-bromocyclohexane
Here is a table with a few of the alkynyl substituents:
Name
|
Molecule
|
Ethynyl
|
-C?CH
|
2- Propynyl
|
-CH2C?CH
|
2-Butynyl
|
-CH3C?CH2CH3
|
Reference
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th Edition. New York: W. H. Freeman & Company, 2007.
Problems
Name or draw out the following molecules:
1. 4,4-dimethyl-2-pentyne
2. 4-Penten-1-yne
3. 1-ethyl-3-dimethylnonyne
Alkyne 1
Introduction

Here are the molecular formulas and names of the first ten carbon straight chain alkynes.
Name
|
Molecular Formula
|
|---|---|
C2H2
| |
Propyne
|
C3H4
|
1-Butyne
|
C4H6
|
1-Pentyne
|
C5H8
|
1-Hexyne
|
C6H10
|
1-Heptyne
|
C7H12
|
1-Octyne
|
C8H14
|
1-Nonyne
|
C9H16
|
1-Decyne
|
C10H18
|
The more commonly used name for ethyne is acetylene, which used industrially.
Alkyne
Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the empirical formula of CnH2n−2 . They are unsaturated hydrocarbons. Like alkenes have the suffix –ene, alkynes use the ending –yne; this suffix is used when there is only one alkyne in the molecule. If a molecule contains both a double and a triple bond, the carbon chain is numbered so that the first multiple bond gets a lower number. If both bonds can be assigned the same number, the double bond takes precedence. The molecule is then named "n-ene-n-yne", with the double bond root name preceding the triple bond root name (e.g. 2-hepten-4-yne).
Subscribe to:
Comments (Atom)
Etea Result
To See Etea result, Download The app. Etea Result
-
Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the empirical formula of C...
-
EXAMPLE 10.1.1 Give the common and IUPAC names for each compound. CH 3 CH 2 CH 2 Br (CH 3 ) 2 CHCl SOLUTION The alkyl group (CH...
-
To See Etea result, Download The app. Etea Result
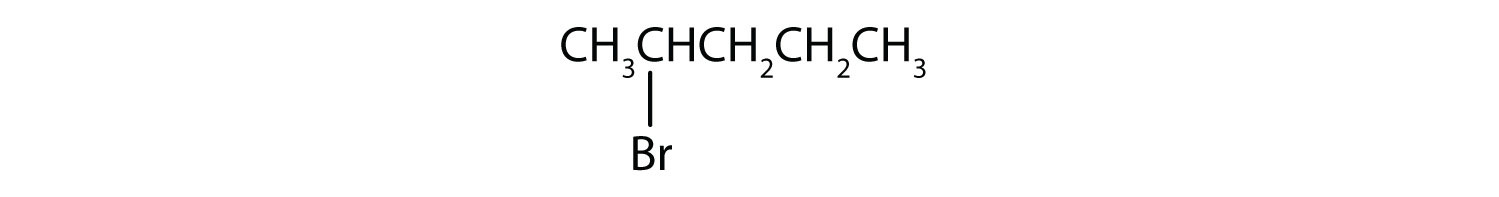
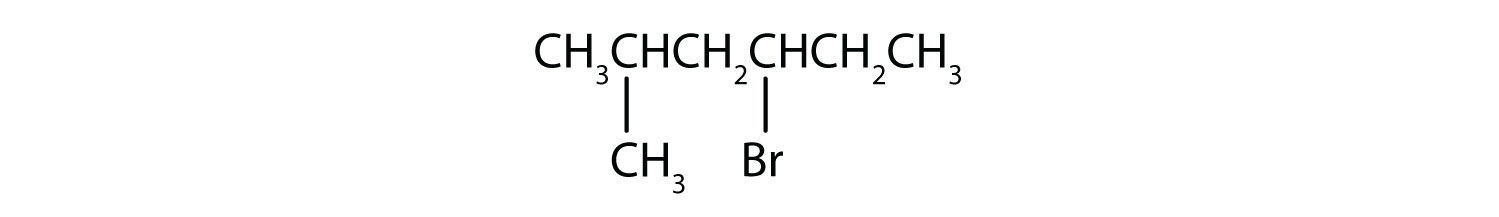
.png?revision=1&size=bestfit&width=322&height=126)
.png?revision=1&size=bestfit&width=191&height=81)