EXAMPLE 10.1.1
Give the common and IUPAC names for each compound.
- CH3CH2CH2Br
- (CH3)2CHCl
SOLUTION
- The alkyl group (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.
- The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the middle carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC name, the Cl atom (prefix chloro-) attached to the middle (second) carbon atom of a propane chain results in 2-chloropropane.
EXERCISE
Give common and IUPAC names for each compound.
- CH3CH2I
- CH3CH2CH2CH2F
Example 10.1.2
Give the IUPAC name for each compound.
SOLUTION
- The parent alkane has five carbon atoms in the longest continuous chain; it is pentane. A bromo (Br) group is attached to the second carbon atom of the chain. The IUPAC name is 2-bromopentane.
- The parent alkane is hexane. Methyl (CH3) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. Listing the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
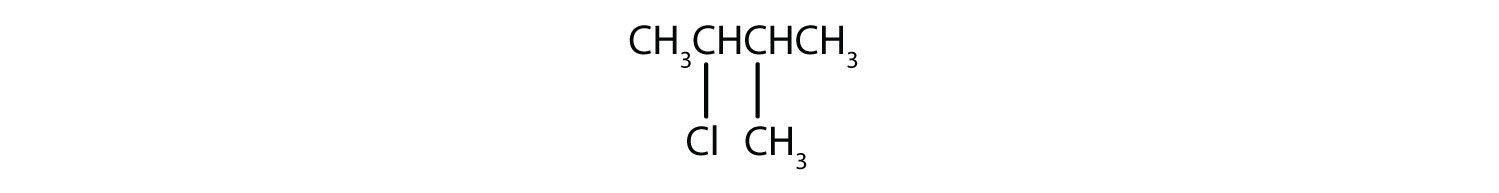
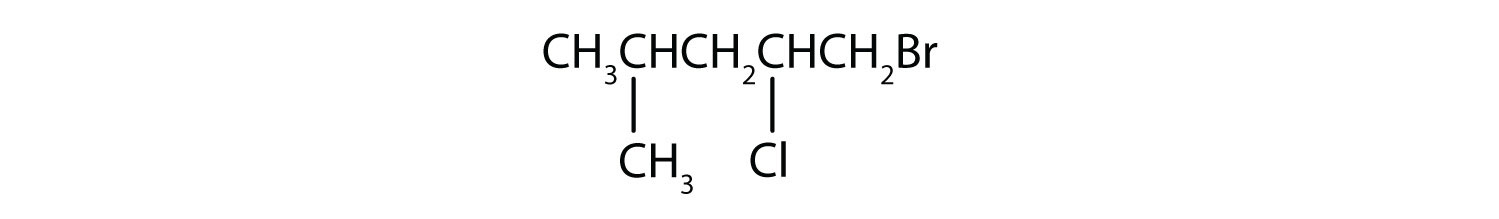
EXERCISE
Give the IUPAC name for each compound.
The haloalkanes, also known as alkyl halides, are a group of chemical compounds comprised of an alkane with one or more hydrogens replaced by a halogen atom (fluorine, chlorine, bromine, or iodine). There is a fairly large distinction between the structural and physical properties of haloalkanes and the structural and physical properties of alkanes. As mentioned above, the structural differences are due to the replacement of one or more hydrogens with a halogen atom. The differences in physical properties are a result of factors such as electronegativity, bond length, bond strength, and molecular size.
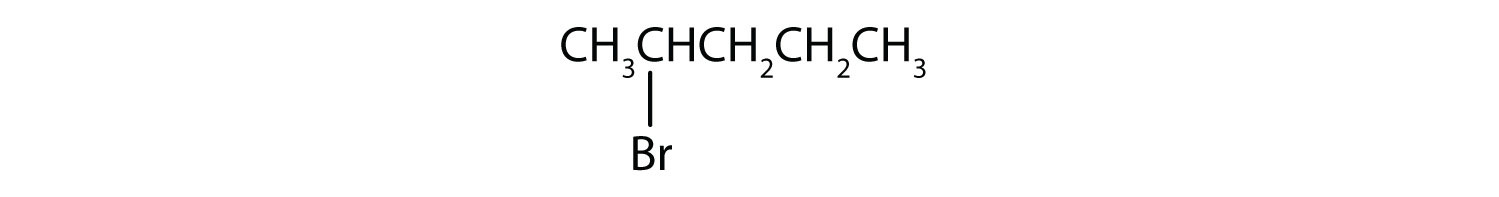
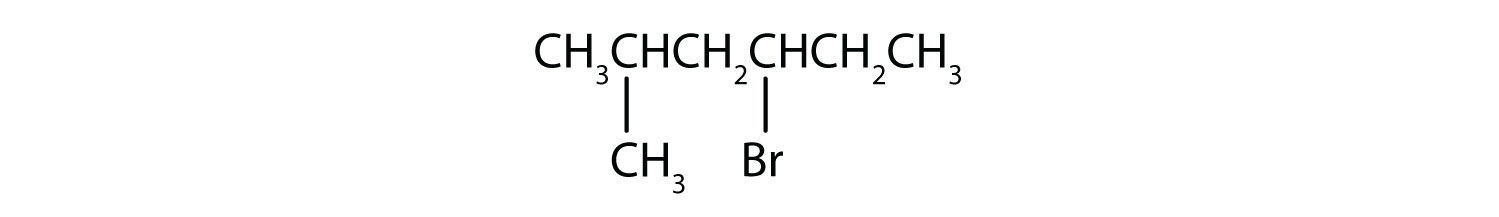
No comments:
Post a Comment