Alkyl halides are also known as haloalkanes. This page explains what they are and discusses their physical properties. alkyl halides are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). We will only look at compounds containing one halogen atom. For example:
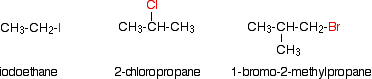
alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. There are some chemical differences between the various types.
Primary alkyl halides
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group.Some examples of primary alkyl halides include:
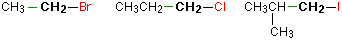
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the halogen. There is an exception to this: CH3Br and the other methyl halides are often counted as primary alkyl halides even though there are no alkyl groups attached to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. Examples:
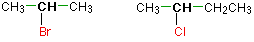
Tertiary alkyl halides
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
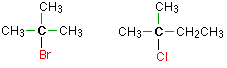
The Learning Objective is to name halogenated hydrocarbons given formulas and write formulas for these compounds given names.
Many organic compounds are closely related to the alkanes. As we noted in Section 12.7, alkanes react with halogens to produce halogenated hydrocarbons, the simplest of which have a single halogen atom substituted for a hydrogen atom of the alkane. Even more closely related are the cycloalkanes, compounds in which the carbon atoms are joined in a ring, or cyclic fashion.
The reactions of alkanes with halogens produce halogenated hydrocarbons, compounds in which one or more hydrogen atoms of a hydrocarbon have been replaced by halogen atoms:

The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by number indicating the substituent’s location. The prefixes are fluoro-, chloro-, bromo-, and iodo-. Thus CH3CH2Cl has the common name ethyl chloride and the IUPAC name chloroethane. Alkyl halides with simple alkyl groups (one to four carbon atoms) are often called by common names. Those with a larger number of carbon atoms are usually given IUPAC names.
No comments:
Post a Comment