EXAMPLE 1
In this diagram this is a cis conformation. It has both the substituents going upward. This molecule would be called (cis) 5-chloro-3-heptene.)
Trans would look like this
v. On the other hand if there are 3 or 4 non-hydrogen different atoms attached to the alkene then use the E, Z system.
E (entgegen) means the higher priority groups are opposite one another relative to the double bond.
Z (zusammen) means the higher priority groups are on the same side relative to the double bond.
(You could think of Z as Zame Zide to help memorize it.)
EXAMPLE 2
Solution
In this example it is E-4-chloro-3-heptene. It is E because the Chlorine and the CH2CH3 are the two higher priorities and they are on opposite sides.
vi. A hydroxyl group gets precedence over th double bond. Therefore alkenes containing alchol groups are called alkenols. And the prefix becomes --enol. And this means that now the alcohol gets lowest priority over the alkene.
vii. Lastly remember that alkene substituents are called alkenyl. Suffix --enyl.
Here is a chart containing the systemic name for the first twenty straight chain alkenes.
| Name | Molecular formula |
| Ethene | C2H4 |
| Propene | C3H6 |
| Butene | C4H8 |
| Pentene | C5H10 |
| Hexene | C6H12 |
| Heptene | C7H14 |
| Octene | C8H16 |
| Nonene | C9H18 |
| Decene | C10H20 |
| Undecene | C11H22 |
| Dodecene | C12H24 |
| Tridecene | C13H26 |
| Tetradecene | C14H28 |
| Pentadecene | C15H30 |
| Hexadecene | C16H32 |
| Heptadecene | C17H34 |
| Octadecene | C18H36 |
| Nonadecene | C19H38 |
| Eicosene | C20H40 |
Did you notice how there is no methene? Because it is impossible for a carbon to have a double bond with nothing.
Geometric Isomers
Double bonds can exist as geometric isomers and these isomers are designated by using either the cis / trans designation or the modern E / Z designation.
cis Isomers
.The two largest groups are on the same side of the double bond.
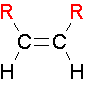
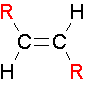
trans Isomers
...The two largest groups are on opposite sides of the double bond.
E/Z nomenclature
E = entgegan ("trans") Z = zusamen ("cis")
Priority of groups is based on the atomic mass of attached atoms (not the size of the group). An atom attached by a multiple bond is counted once for each bond.
fluorine atom > isopropyl group > n-hexyl group
deuterium atom > hydrogen atom
-CH2-CH=CH2 > -CH2CH2CH3
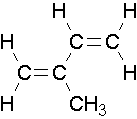
EXAMPLE 3
Try to name the following compounds using both conventions...
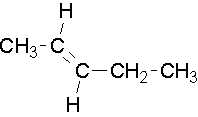
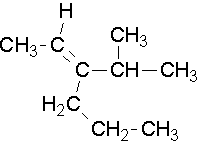
Common names
Remove the -ane suffix and add -ylene. There are a couple of unique ones like ethenyl's common name is vinyl and 2-propenyl's common name is allyl. That you should know are...
- vinyl substituent H2C=CH-
- allyl substituent H2C=CH-CH2-
- allene molecule H2C=C=CH2
- isoprene
Endocyclic Alkenes
Endocyclic double bonds have both carbons in the ring and exocyclic double bonds have only one carbon as part of the ring.
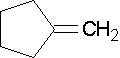
Cyclopentene is an example of an endocyclic double bond.

Methylenecylopentane is an example of an exocyclic double bond.
Name the following compounds...
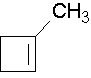
1-methylcyclobutene. The methyl group places the double bond. It is correct to also name this compound as 1-methylcyclobut-1-ene.
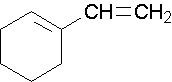
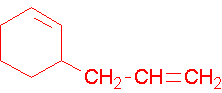
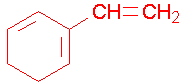
1-ethenylcyclohexene, the methyl group places the double bond. It is correct to also name this compound as 1-ethenylcyclohex-1-ene. A common name would be 1-vinylcyclohexene.
Try to draw structures for the following compounds...
- 2-vinyl-1,3-cyclohexadiene
No comments:
Post a Comment