Note that the numbering of "2-4" above yields a molecule with two double bonds separated by just one single bond. Double bonds in such a condition are called "conjugated", and they represent an enhanced stability of conformation, so they are energetically favored as reactants in many situations and combinations.
| Alkane | CnH2n+2 | This is the maximum H/C ratio for a given number of carbon atoms. |
|---|---|---|
| Alkene | CnH2n | Each double bond reduces the number of hydrogen atoms by 2. |
The parent structure is the longest chain containing both carbon atoms of the double bond. The two carbon atoms of a double bond and the four atoms attached to them lie in a plane, with bond angles of approximately 120° A double bond consists of one sigma bond formed by overlap of sp2 hybrid orbitals and one pi bond formed by overlap of parallel 2 p orbitals
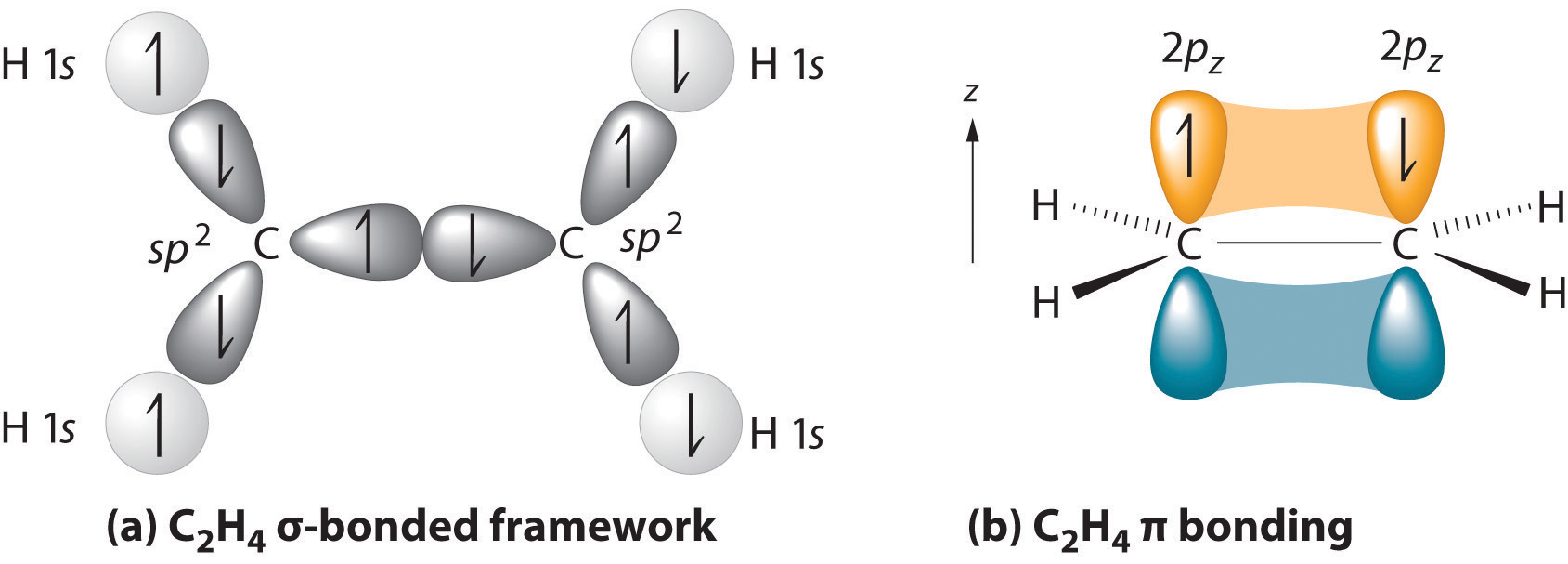
Figure 1: (a) The σ-bonded framework is formed by the overlap of two sets of singly occupied carbon sp2 hybrid orbitals and four singly occupied hydrogen 1s orbitals to form electron-pair bonds. This uses 10 of the 12 valence electrons to form a total of five σ bonds (four C–H bonds and one C–C bond). (b) One singly occupied unhybridized 2pz orbital remains on each carbon atom to form a carbon–carbon π bond. (Note: by convention, in planar molecules the axis perpendicular to the molecular plane is the z-axis.)
The molecular formula of a hydrocarbon provides information about the possible structural types it may represent. For example, consider compounds having the formula C5H8. The formula of the five-carbon alkane pentane is C5H12 so the difference in hydrogen content is 4. This difference suggests such compounds may have a triple bond, two double bonds, a ring plus a double bond, or two rings. Some examples are shown here, and there are at least fourteen others!
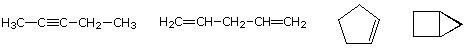
IUPAC Rules for Alkene and Cycloalkene Nomenclature
- The ene suffix (ending) indicates an alkene or cycloalkene.
- The longest chain chosen for the root name must include both carbon atoms of the double bond.
- The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
- The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator. If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator number.
- In cycloalkenes the double bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule.
- Substituent groups containing double bonds are:
H2C=CH– Vinyl group
H2C=CH–CH2– Allyl group
No comments:
Post a Comment